In this article we’re going to look at the Sensible and Latent Heat Transfer Equation for Air. This is useful when trying to determine either of the three variables, Btu’s, CFM or Delta in Enthalpy. When you know two of these values you can determine the remaining missing value.
If you prefer to watch the YouTube Video of this presentation than scroll to the bottom.
Sensible and Latent Heat Transfer Equation for Air
q = m ∆h
q = CFM x 0.075 lb/ft2 x 60 min/hour x ∆h
q = CFM x 4.5 x ∆h
CFM is ft3/minute
M is the overall mass flow rate of air
h is the Enthalpy (Btu/lb.)
We’ll need to look at a Psychrometric Chart to gather the information needed for this calculation. This is also easily done using software, but it’s best to know how the software arrives at these values.
We’ll begin by calculating for the BTU’s when given the CFM, and the temperature and relative humidity of the Entering and Leaving Air conditions. Remember we’re dealing with both sensible and latent heat. That means were dealing with two factors, the sensible or change in temperature and the latent, the change in state, in this case it will be moisture condensing on a cooling coil.
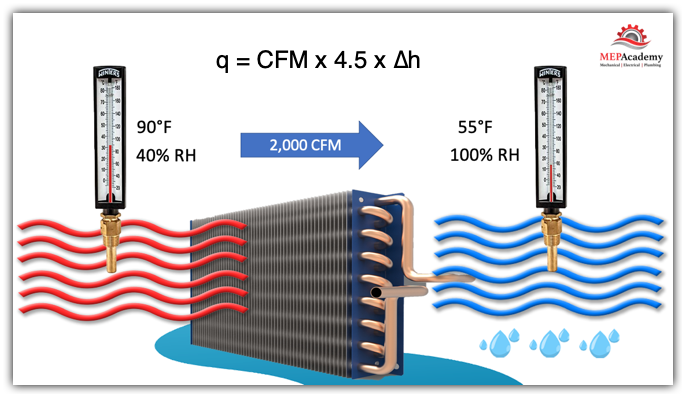
Looking at this chilled water coil we see that there is 2,000 Cubic Feet per minute of air flowing through this coil and the temperature of the Entering air is 90°F and 40% relative humidity with the Leaving Air at 55°F and 100% relative humidity.
Example:
2,000 CFM of outdoor air (Ventilation Air) at 90°F and 40% relative humidity (RH) is drawn through a cooling coil that brings the conditions to 55°F and 100% relative humidity (RH)
Step #1 – Plot the given conditions on the Psychrometric Chart
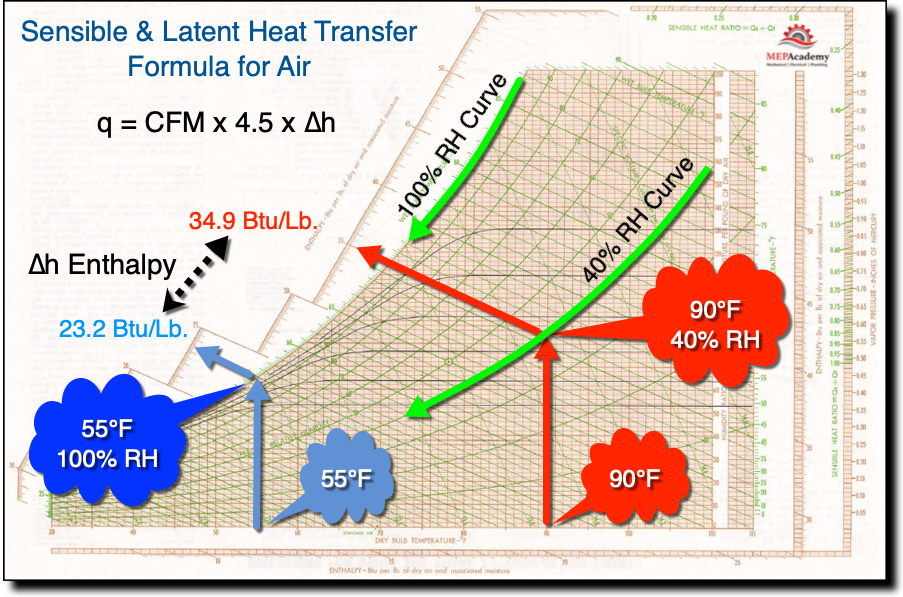
Step #2 – Get the h1 and h2 values from the Psychrometric Chart 34.9 and 23.2
Step #3 – Enter all values into equation.
q = CFM x 4.5 x ∆h
q = 2,000 x 4.5 x 11.7 = 105,300 Btu/hour
With this information we can solve for how many btu’s are being supplied to the air.
Step one is to plot the entering and leaving air conditions on a psychrometric chart to determine the enthalpy. For the entering air conditions of 90°F and 40% relative humidity (RH), we enter here along the horizontal part of the chart that reflects temperature and then we go vertical straight up from there until we hit our 40% Relative Humidity line and then diagonally to the left until we intersect the enthalpy line. Here we get we get an enthalpy of 34.9, and then we plot the leaving air conditions of 55°F and 100% relative humidity we get an enthalpy of 23.2. 34.9 – 23.2 gives us an enthalpy of 11.7 Btu/lb.
Step two is to put all the known values into our formula and make the calculation.
We have our formula of q = CFM x 4.5 x ∆h
Now we enter our values, we get
q = 2,000 x 4.5 x 11.7 = 105,300 Btu/hour
Now we quickly explain where the value of 4.5 in the calculation is derived from. First, we have the weight of Air at 0.075 pounds per Ft3, then we have the conversion of 60 minutes into hours. This is what that looks like
0.075 lb/ft3 x 60 min/hour = 4.5
With all these units, we can see which units of value remain by crossing out those that are eliminated in the formula as such
q = 2,000 Ft3/minute x 0.075 lb/ft3 x 60 min/hour x 11.7 Btu/lb = 105,300 Btu/hour
Checkout these similar Videos related to sensible heat:







