In this article we’re going to look at the Sensible Heat Transfer Equation for Water. This is useful when trying to determine either of the three variables, Btu’s, GPM or Delta-T. When you know two of these values you can determine the remaining missing value.
If you prefer to watch the YouTube Video of this presentation than scroll to the bottom.
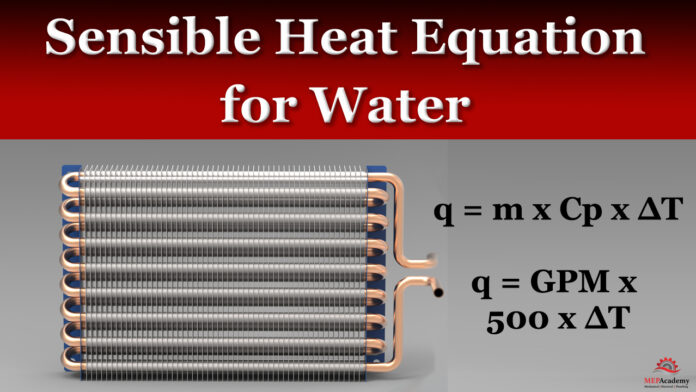
Sensible Heat Transfer Equation for Water
q = m x Cp x ∆T
q = GPM x 8.34 lb/gallon x 60 min/hour x 1 btu/lb°F x ∆T
q = GPM x 500 x ∆T
GPM is gallons/minute
m is the overall mass flow rate of water
Cp is the specific heat of water 1 btu/lb°F
We’ll begin by calculating for the BTU’s when given the GPM and temperatures of the supply and return water. Remember we’re dealing with sensible heat only. That means there is no latent heat which involves a change of state, like water becoming steam. All were doing is changing the temperature of water and not its state.
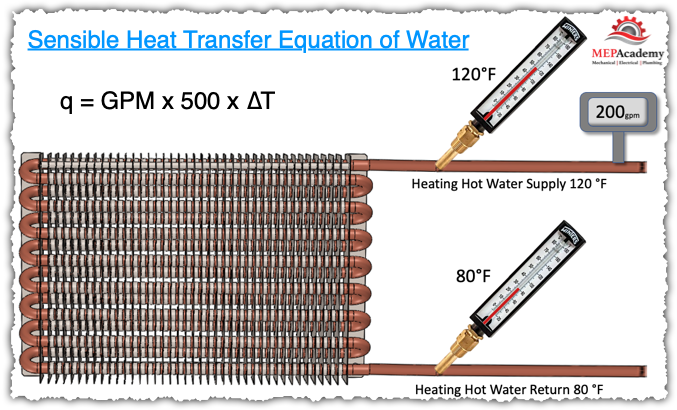
Example:
200 GPM water enters a coil at 120°F and leaves at 80°F
Step #1 – Determine the ∆T (120°F – 80°F = 40°F)
Step #2 – Enter all values into equation.
q = GPM x 500 x ∆T
q = 200 x 500 x 40 = 4,000,000 Btu/hour
Looking at this heating hot water coil we see that there is 200 gallons per minute of water flowing through this coil and the temperature of the heating hot water supply is 120°F and the leaving heating hot water is 80°F.
With this information we can solve for how many btu’s are being supplied to this coil.
Step one is to subtract the Leaving Water temperature of 80°F from the Entering Water temperature of 120°F to arrive at the Temperature difference or Delta-T. 120°F – 80°F, gives us a 40°F Delta-T.
Step two is to put all the known values into our formula and make the calculation.
We have our formula of q = GPM x 500 x ∆T
Now we enter our values, we get
q = 200 x 500 x 40 = 4,000,000 Btu/hour
Now we quickly explain where the value of 500 in the calculation is derived from. First, we have the weight of water at 8.34 pounds per gallon, then we have the conversion of minutes into hours, and lastly the specific heat of water at 1 btu/lb°F. This is what that looks like
8.34 lb/gallon x 60 min/hour x 1 btu/lb°F = 500
With all these units, we can see which units of value remain by crossing out those that are eliminated in the formula as such
Q = 200 gallons/minute x 8.34 lb/gallon x 60 min/hour x 1 btu/lb°F x 40°F = Btu/Hour
Checkout our other video on Calculating Sensible Heat for Air or Calculating Sensible and Latent Heat.