Why use steam for heating? Steam is an excellent medium for heating due to its unique properties, particularly its specific volume and latent heat of vaporization. Here’s an explanation of why these properties make steam beneficial for heating.
If you prefer to watch the video of this presentation, then scroll to the bottom.
Specific Volume of Steam
Specific volume is the volume occupied by a unit mass of a substance, in this case steam.
Steam has a significantly larger specific volume compared to liquid water. This means that for the same mass, steam occupies a much larger space. When steam gives up its heat and condenses into water, it undergoes a dramatic reduction in volume, because water occupies much less space.
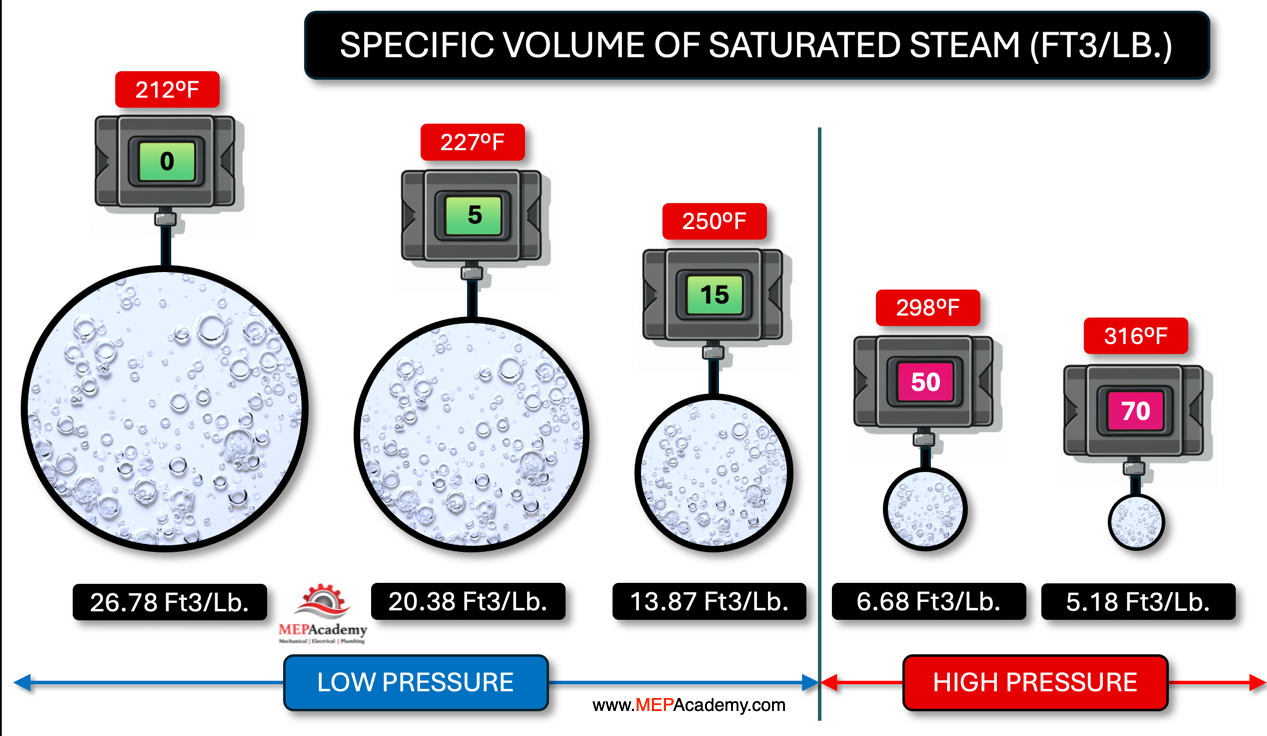
Here we have 1 pound of steam at different gauge pressures. At 0 PSIG our specific volume is the greatest at 26.78 cubic feet per pound of steam. As we increase the pressure on the same pound of steam the volume gets smaller, and smaller. At 15 PSIG the volume is about half of that at 0 PSIG, and if we increase the pressure to 50 PSIG the volume decreases again by about half to 6.68 cubic feet per pound. Boilers are rated by their pressure, with low pressure considered 15 PSIG and less, and pressures over that are considered high pressure boilers.
We can see that if we drop the pressure from 50 psig to 15 psig, the same amount of steam now requires twice as much space or volume. By increasing the steam pressure, we can squeeze the same amount of steam into a smaller space. Now less look at how much energy steam holds at these different pressures.
Latent Heat of Vaporization for Dry Steam
The latent heat of vaporization is the amount of heat required to convert a unit mass of a liquid into a vapor without a temperature change. Steam holds more energy per pound than water. If we first look at water at 32 degrees Fahrenheit and the energy it takes to get that 1 pound of water to the boiling point of 212 degrees Fahrenheit, and then convert that pound of water to vapor we’ll understand the differences.

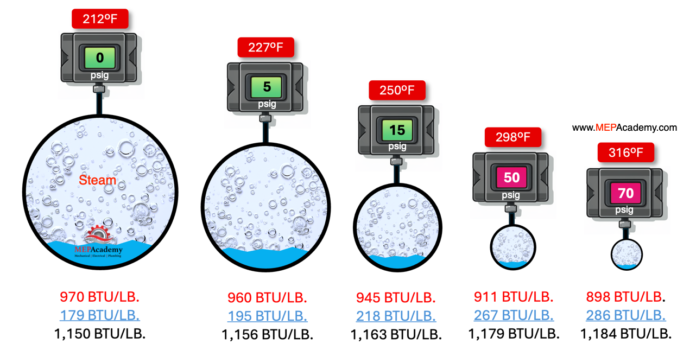
A BTU is the amount of heat required to raise 1 pound of water, 1 degree Fahrenheit. This would require 180 BTUs to raise our water at 32 degrees to 212 degrees Fahrenheit. This means our 1 pound of liquid water at 212 degrees Fahrenheit holds 180 btu’s. We would then need approximately 970 BTU to convert the water at 212 degrees Fahrenheit to vapor at 212 degrees Fahrenheit, there is no change in temperature, just a change of state from water to vapor. The vapor holds 970 Btu’s, while the water holds only 180 Btu’s. This is one of the big advantages of using steam.
Steam carries a large amount of energy due to its high latent heat of vaporization. When steam condenses back into water on the surface of a heat exchanger, it releases this substantial amount of energy, which can be used for heating purposes. This energy release is highly efficient, making steam an effective medium for transferring heat.
Why Use Steam for Heating
The enthalpy of steam does not significantly change with an increase in pressure, which means that the total energy content (including both sensible heat and latent heat) of steam remains relatively constant across different pressures. This characteristic indicates that the efficiency of steam as a heat transfer medium is not primarily due to changes in its enthalpy with pressure.
Instead, the primary advantage of using steam lies in its high latent heat of vaporization and the efficient heat transfer during condensation. These properties enable steam to transfer large amounts of energy quickly and effectively, making it a preferred choice for heating applications despite the relatively stable enthalpy across varying pressures.
As the pressure of steam increases, its specific volume significantly decreases, meaning that the steam becomes denser and occupies less space per unit mass. This reduction in specific volume with higher pressure allows for the use of smaller diameter piping to transport the same amount of steam energy.
Smaller pipes require less material, which reduces the overall material costs. Additionally, smaller piping is easier and quicker to install, leading to lower labor costs. This efficiency in piping size and installation makes steam systems economically advantageous in industrial and commercial applications.
Efficient Heat Transfer using Steam
When steam contacts a cooler surface, it condenses rapidly, releasing a large amount of heat almost instantaneously. This rapid condensation makes steam an excellent medium for delivering heat quickly and efficiently.
Steam provides uniform heating as it condenses at a constant temperature. This is particularly advantageous in processes requiring consistent temperature control.
Ease of Transport and Control
Due to its gaseous state, steam can be easily transported through pipes over long distances without significant heat loss. This makes it ideal for centralized heating systems where the heat source is distant from the application point.
Steam systems are relatively easy to control using valves and other mechanisms, allowing for precise regulation of heat delivery to different parts of a building or process.
Economic and Practical Considerations
Steam heating systems are often cost-effective, both in terms of initial setup and operational costs, especially in large-scale applications like commercial buildings and industrial processes.
Steam can be used in a variety of heating applications, from space heating in buildings to process heating in industries.
In summary, steam’s high latent heat of vaporization and large specific volume, combined with its efficient heat transfer capabilities and ease of transport and control, make it a highly effective and versatile medium for heating applications.